Intensive treatment approaches are required for adult Burkitt lymphoma (BL), although a univocal standard of care still does not exist. The use of frontline autologous transplantation is debated.
Between 2004 and 2020, 50 HIV-negative BL patients were treated with the Berlin-Frankfurt-Münster (BFM) protocol at our institution. Treatment plan consisted of 3 blocks, A (ifosfamide, vincristine, methotrexate, etoposide, cytarabine), B (vincristine, cyclophosphamide, methotrexate, doxorubicin) and C (vindesine, methotrexate, etoposide, cytarabine), each repeated twice, every 28 days. Patients elder than 60 years did not receive block C, thus blocks A and B were repeated 3 times. Rituximab was given at day 1 each block. Intrathecal prophylaxis was given once per each block. Autologous stem cells were reinfused (ASCT) at the end of the 6-blocks after BEAM (carmustine, etoposide, cytarabine, melphalan) conditioning, when feasible.
Median age at onset was 38 years (range 16-72); 38 patients were male and 12 female. Stage III-IV disease was observed in 82% of cases; bulky disease occurred in 44% of the patients, with B-symptoms in 38%. Two patients did not receive rituximab because of adverse reaction and early death. Intrathecal prophylaxis was given in 96% of patients. Stem cell harvest was performed in 70% of patients, who all received a subsequent ASCT. The full 6-blocks treatment was completed in 70% of the patients; 8% received 5 cycles and 22% received ≤ 3 cycles. Early treatment interruption occurred because of disease progression (12%), toxicity (8%), death (4%) or other causes (4%).
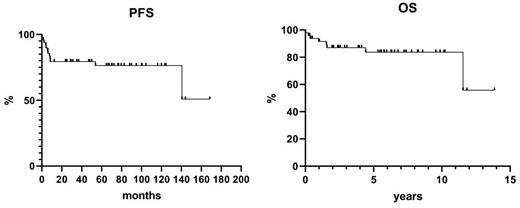
The overall response rate was 74%, with a complete response rate of 60%. Three patients could not be evaluated because of early progression/death. Ten-year overall survival and progression-free survival were 83.7% and 76.0%, respectively, with both curves exhibiting a plateau (Figure). Ten-year disease-free survival was 80.3%. Eight patients had died because of disease progression (3 patients), infection or sepsis (4 patients) or cardiac arrest (1 patient). Grade 3-4 neutropenia, thrombocytopenia, anemia and mucositis were seen in 96%, 60%, 32% and 24% of patients. Infections occurred in 60% of patients; grade 4 and fatal sepsis in 14% and 8% of cases.
Intensive treatment according to BFM protocol, with rituximab and ASCT, appears feasible, safe and highly effective in adult patients with BL, as confirmed by long-term survival rates.
Disclosures
Casadei:Roche: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Speakers Bureau; Lilly: Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite-Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Zinzani:BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSAPHARMA: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SECURA BIO: Membership on an entity's Board of Directors or advisory committees; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ROCHE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC THERAPEUTICS: Membership on an entity's Board of Directors or advisory committees; ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CELLTRION: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; JANSSEN-CILAG: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GILEAD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; INCYTE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KYOWA KIRIN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SANDOZ: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal